Melen Research Group
Advancing Main Group Chemistry
Metal-Free Catalysis
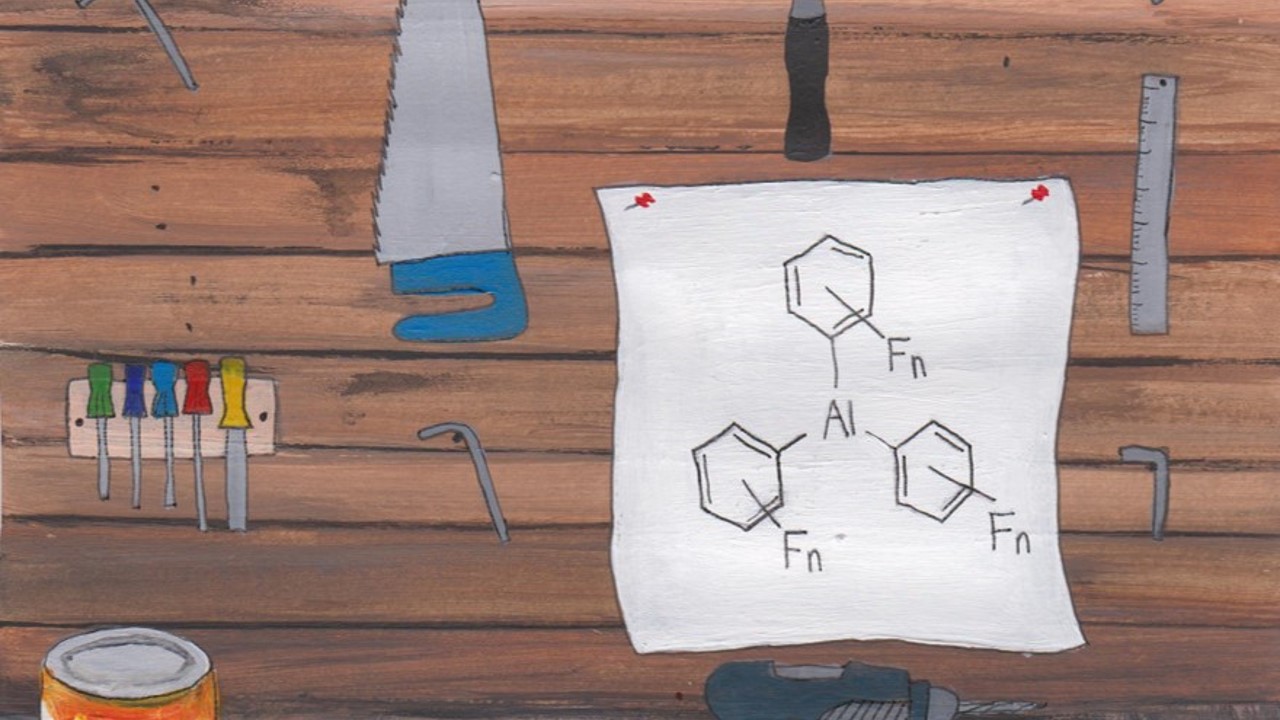
Catalysis plays a pivotal role in maintaining the quality of everyday life worldwide as over 85% of chemical products are generated through catalytic methods. Transition metals have dominated these processes, however many of the most active catalysts are composed of precious metals which are expensive and toxic. The latter is particularly significant for consumer products that are taken into the body (e.g. pharmaceuticals). One focus of our research has been the development of non-transition metal catalysts through modulating the steric and electronic demands of ligands. This has led to a series of p-block catalysts based upon boron, aluminium, phosphorus and arsenic. In addition, we have developed chiral main group catalysts to enable enantioselective catalysis. We have also demonstrated that several of these catalysts can be used to mimic precious metals in catalytic reactions, and perhaps more importantly, sometimes show complementary reactivity or selectivity. My research has shown that p-block catalysts can be employed in hydrogenation, hydroboration, cyclisation, alkylation, and carbene transfer reactions. The products of these catalytic reactions are omnipresent motifs in numerous biologically active compounds.
Selected recent publications:
Guerzoni, M.G., van Ingen, Y., Babaahmadi, R., Wirth, T., Richards,* E., Melen,* R.L., “An un-forgotten classic: the nitro-Mannich reaction between nitrones and silyl nitronates catalysed by B(C6F5)3”, Chem. Sci., 2024, 15, 2648.
Dasgupta, A., Stefkova, K., Babaahmadi, B., Gierlichs, L.J., Ariafard,* A., Melen,* R.L., “Triarylborane-catalysed alkenylation reaction of aryl esters with diazo compounds”, Angew. Chem. Int. Ed., 2020, 59, 15492.
Dasgupta, A., Babaahmadi, R., Slater, B., Yates, B.F., Ariafard, A., Melen,* R.L., “Borane-catalyzed stereoselective C–H insertion, cyclopropanation, and ring-opening reactions”, Chem, 2020, 6, 2364.
Santi, M., Ould, D.M.C., Wenz, J., Soltani, Y., Melen,* R.L., Wirth,* T., “Metal-free tandem rearrangement/ lactonization: access to 3,3-disubstituted benzofuran-2-(3H)-ones”, Angew. Chem. Int. Ed., 2019, 58, 7861.
Single or Double? Radical Reactions of the p-Block Elements
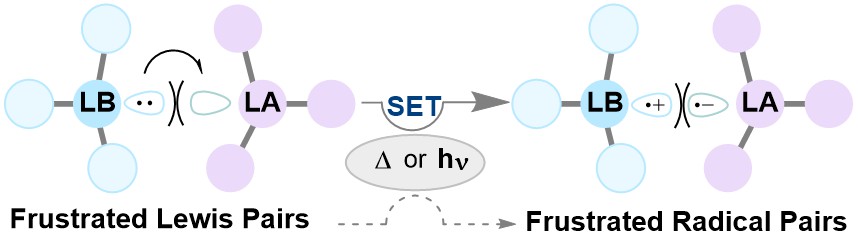
Reaction mechanisms in p-block chemistry are currently dominated by two-electron processes, however my research has shown that single-electron transfer pathways can offer an alternative, lower-energy pathway to bond formation. Understanding this reactivity is paramount for the continued development of synthetic chemistry, and in the Melen group we have collaborated with the Browne (UCL) and Porch (Cardiff, Engineering) to understand how metal free systems known as “Frustrated Lewis Pairs” (FLPs) activate dihydrogen. We have also investigated the utilisation of FLPs in C–H functionalisation reactions. Using detailed mechanistic studies including computational chemistry, NMR and EPR spectroscopy, our work has challenged the existing knowledge of metal-free transformations through double-electron transfer, to suggest a low-energy single-electron process akin to 1st-row metals. Providing complementarity to transition metal reactivity, these radical reactions of the p-block elements has rapidly gathered interest from the scientific community, for which there are ample opportunities for future development.
Selected recent publications:
Yu, C., Leitch, J.A., Gierlichs, L., Das, S., Porch,* A., Melen,* R.L., Browne,* D.L., “The use of microwave dielectric spectroscopy for the in actu assessment of frustrated Lewis pair encounter complexes”, J. Am. Chem. Soc., 2024, 146, 19809.
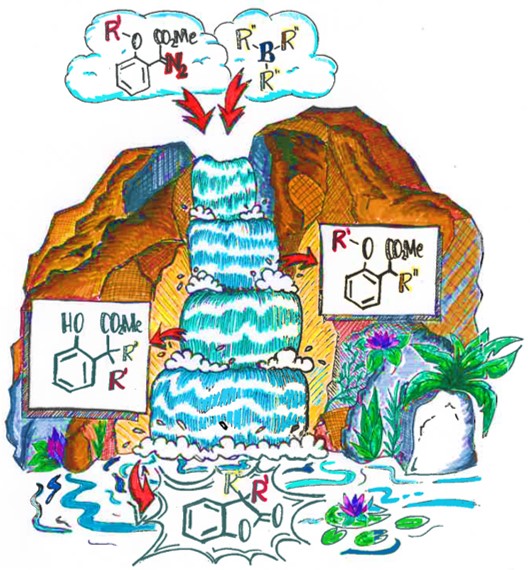
Pramanik, M., Das, S., Babaahmadi, R., Pahar, S., Wirth,* T., Richards,* E., Melen,* R.L., “B(C6F5)3-Catalysed chemo- and regioselective C–H chalcogenation of arenes and heteroarenes”, Chem, 2024, 10, 2901.
van der Zee, L., Pahar, S., Richards,* E., Melen,* R.L., Slootweg, J.C., “Insights into single-electron transfer processes in frustrated Lewis pair chemistry and related donor-acceptor systems in main group chemistry", Chem. Rev., 2023, 123, 9653.
Dasgupta, A., Stefkova, K., Babaahmadi, B., Yates, B.F., Buurma, N.J., Richards, E., Ariafard, A., Melen,* R.L., “Site selective Csp3–Csp / Csp3–Csp2 cross coupling reactions using frustrated Lewis pairs”, J. Am. Chem. Soc., 2021, 143, 4451.
Compound Characterisation
Elemental analysis, awarded the Nobel Prize in 1923, is a common technique used to analyse the purity of chemical samples by measuring the percentage carbon, hydrogen, and nitrogen. Many journals stipulate that C/H/N values must not deviate from the compound’s formula by more than 0.4%. In a study joint with La Trobe (Dutton, Kuveke), Dalhousie (Chitnis) and Baylor (Martin) we showed that the standard journal requirement of ±0.4% for C/H/N values is not statistically sound, as results of pure, air-stable samples can be wildly inconsistent, likely because of differences in calibration across instruments. This has had significant impact with several highlight articles and news items. This has led to a change in the way chemists look at compound purity and several journals (e.g. Wiley) have changed their requirements for compound characterisation.
Published in:
Kuveke,* R.E.H., Barwise, L., van Ingen, Y., Vashisth, K., Roberts, N., Chitnis,* S.S., Dutton,* J.L., Martin,* C.D., Melen,* R.L., “An international study evaluating elemental analysis”, ACS Cent. Sci., 2022, 8, 855.
Industrial sponsors (past and present): AstraZeneca, BP, GEO Speciality Chemicals, Johnson Matthey, Pfizer, Tokyo Ohka Kogyo.
Funding:
Overview
Research in the Melen group focuses on developing and utilising p-block reagents for promoting or catalysing chemical transformations. Not only does our work offer the promise of devising new strategies for “green” chemical synthesis, but it has led to an improved understanding of how element reactivity might be modulated. Below are several key areas of research currently being explored within the Melen research group which is directed towards developing new p-block reagents for synthesis, catalysis, and industrial applications.
Selected publication: